Platform Introduction
The concept of real-world studies (RWE) was first introduced by Williamson and Barrett in 1966 and has been gaining attention in recent years. Data collected in the course of medical device and health care activities. Following the release of the 21st Century Cures Act, the industry believed that RWE would speed up drug approvals while reducing the scientific validity of reviews. Based on these concerns, in December 2016, the FDA published "Real-World Evidencce-What is it and what can it tell us?" in the New England Journal of Medicine, systematically describing the environment and research methods used by RWE to obtain data. In September 2017, the FDA accepted RWE for medical device registration. Currently, many completed or ongoing RWE trials can be retrieved on ClinicalTrials.gov. In the context of the FDA's vigorous promotion, combined with its advantages of wide inclusion, few exclusions and authenticity, RWE will certainly play an important role in accelerating the approval of drugs and medical devices.
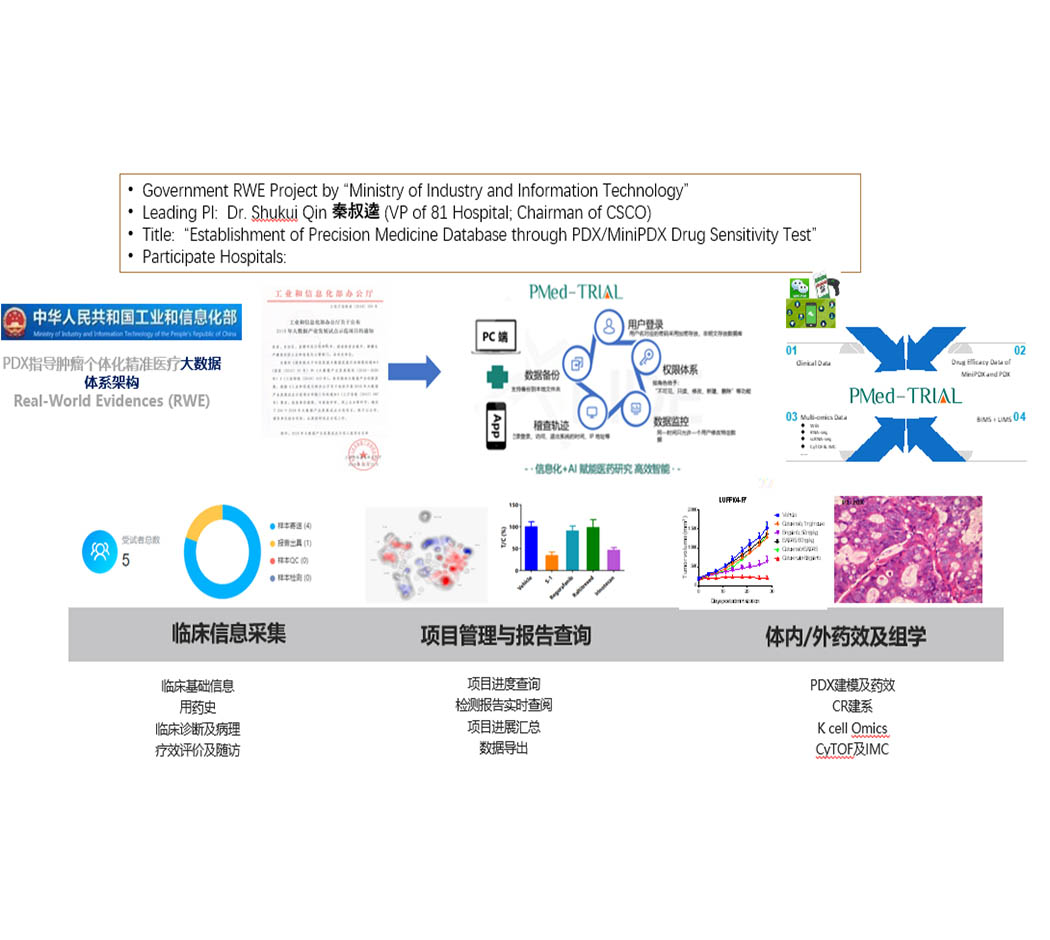
As the main support unit of the 2018 RWE big data project "Establishment and Application Research of PDX Model-based Tumor Individualized Precision Medical Big Data Platform" led by Prof. Qin Shukui, based on the need to build a 10,000-person data-scale Chinese population clinical vitalomics database of major cancer diseases, clinical phenotype database of tumor patients, PDX model and MiniPDX pharmacodynamic phenotype Ltd. has developed a data system for precision medicine and clinical trial refinement management, PMed-Trial, which integrates sponsors, institutions, ethics, departments, CRAs, CRCs and subjects in a comprehensive management platform, aiming to provide a one-stop solution for the whole process of clinical trials.
PMed-Trial is an intelligent data management platform for clinical research, covering clinical trial research and clinical laboratory data, while also enabling real-time project control; providing accurate and powerful data support for drug clinical research, and helping clinics achieve individualized precision medicine for tumors.
System Features
Precision Medicine
Functional tests guide individualized precision medicine for tumors
Comprehensive regulatory compliance
System complies with NMPA and FDA requirements for clinical data collection and ISO data management system
Secure and stable data storage
The system achieves control from various protection dimensions such as role authority management, audit trail, data backup, etc.
Efficient and flexible CRF design
The system can be designed according to different scenarios for CRF, easy access and convenient operation
Document Review Project Management Integration
The system supports process management such as file upload, online preview and project alert
RWE&PMed-Trial Application Scenarios
RWE Real World Research Project
PI-initiated IIT studies
Pre-market PhaseI clinical trials (laboratory component)
Project Process Management
RWE&PMed-Trial partner medical institutions
Zhongshan Hospital of Fudan University
Cancer Hospital of Fudan University
Shanghai Pulmonary Hospital of Tongji University
Dongfang Hospital of Tongji University
Shanghai International Medical Center
Renji Hospital of Shanghai Jiaotong University
China-Japan Union Hospital of Jilin University
Peking Union Medical College Hospital