Introduction to functional testing platform
Functional assays include all tests that monitor the state of tumor activity and are used to guide patient treatment, such as measuring tumor apoptosis by biopsy after in vitro drug exposure. Functional assays differ from molecular or genetic assays in that they utilize unique assays to assess the effectiveness of an intervention directly by observing the phenotypic performance of the intervention. It is direct and simple. Currently, Shanghai Levin's unique functional assay platform mainly includes PDX model library and MiniPDX in vivo drug sensitivity assay platform.
PDX Model Library Introduction
The patient-derived xenograft model (PDX model) is a new generation tumor model established by directly transplanting fresh tumor tissue from patients into immunodeficient mice. This model retains the histological and genetic characteristics of the original tumor, and its pharmacodynamic results are clinically relevant up to 91%. The main research directions using PDX model.
1、PDX modeling: continuously explore more PDX modeling methods for cancer species, improve modeling success rate, and establish standardized SOPs for PDX modeling
2、Preclinical research: using PDX model for drug screening and "re-validation" of marketed drugs
3、Basic clinical research: use PDX model to find new tumor biomarkers, and conduct research on tumor clonal evolution, tumor molecular characteristics, tumor pathogenesis, etc.
4. Clinical application research: PDX model is used for individualized tumor treatment research to provide clinicians with a basis for treatment decisions.
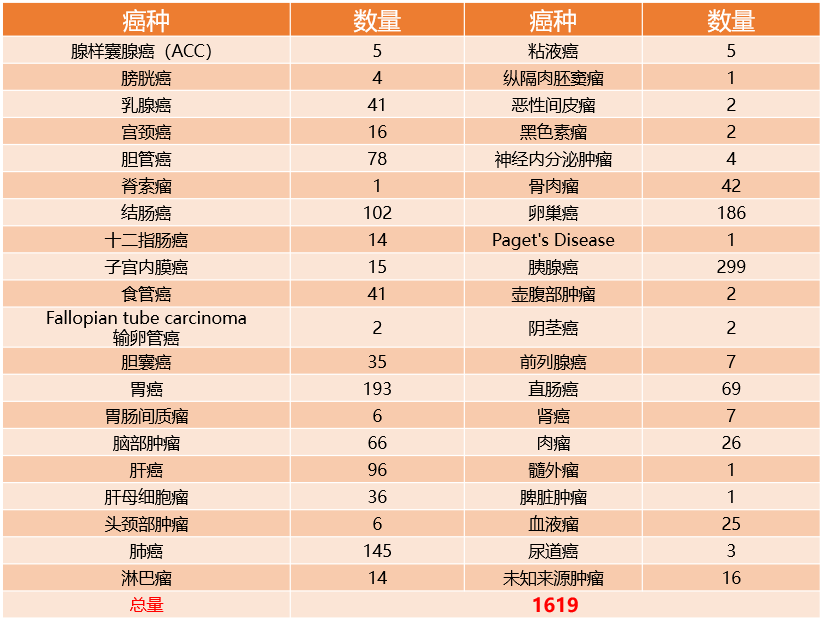
Live bank of resuscitable biological samples
MiniPDX in vivo drug sensitivity testing platform
The MiniPDX in vivo drug sensitivity assay is a new generation functional assay that allows mice to test drugs on behalf of patients. It is an in vivo version of the drug sensitivity test that is performed by digesting the patient's own fresh tumor tissue into a tumor cell suspension in a special capsule device and then implanted subcutaneously in immunodeficient mice.
The assay allows for rapid drug sensitivity testing of multiple drugs and drug combination regimens, allowing for the selection of individualized drug regimens and providing a scientific basis for clinical drug selection.
The PDX model is recognized as the gold standard in the industry, and the correlation between its efficacy test results and clinical practice is as high as 89%, and the validation data of the efficacy of MiniPDX and PDX model shows that the correlation between MiniPDX and PDX model is 92%. Therefore, in addition to providing patients with personalized treatment plans in clinical applications, MiniPDX has great potential for screening new drug indications and evidence-based innovative treatment pathways in the pipeline.
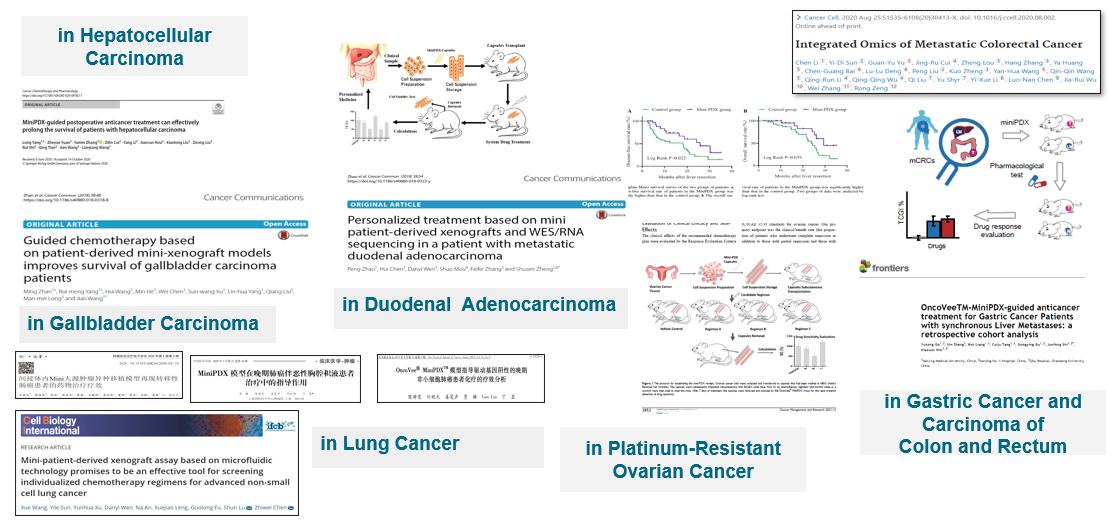
Evidence from MiniPDX clinical studies