Product Introduction
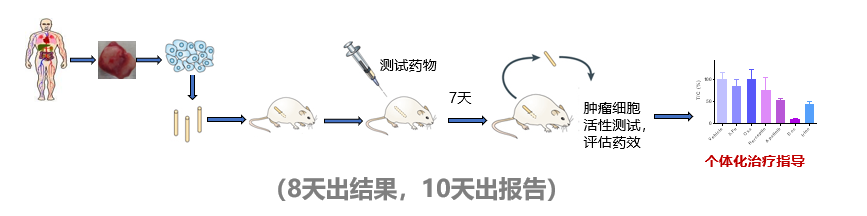
MiniPDX in vivo drug susceptibility test is a new generation of functional testing technology that allows mice to test drugs for patients. It is performed by digesting the patient's own tumor tissue into a tumor cell suspension, placing it into a special capsule, and then implanting it subcutaneously in immunodeficient mice.
This method can quickly conduct drug sensitivity tests on a variety of drugs and combination schemes, thus optimizing individualized drug regimens, and providing a scientific basis for patients' clinical drug selection.
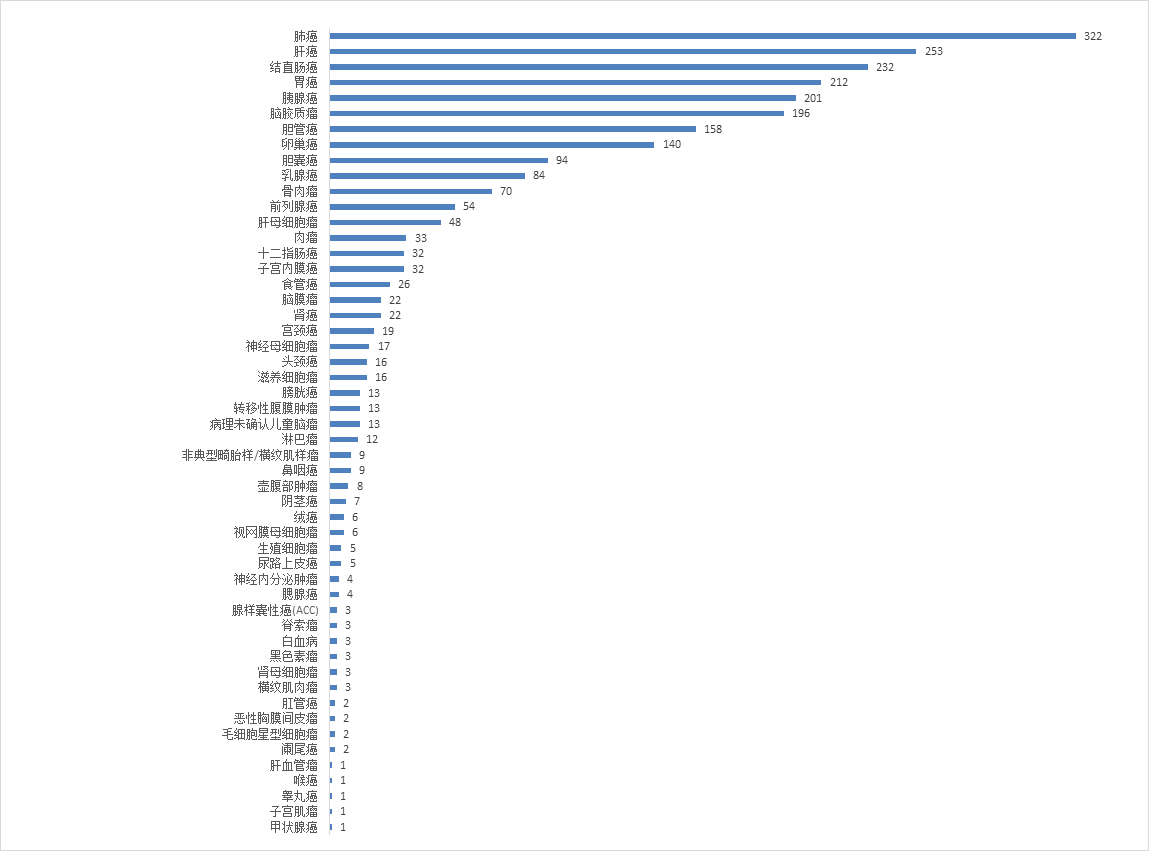
As an industry-recognized gold standard, the PDX model has an 89% correlation between its test results and clinical efficacy. The validation data shows that the correlation between MiniPDX and PDX models is 92%. Therefore, in addition to providing patients with personalized treatment plans, MiniPDX also has great application prospects for the screening of new drug indications and evidence-based innovative treatment pathways.
Several advantages of MiniPDX

High clinical consistency
The consistency of MiniPDX™ drug susceptibility test and traditional PDX is as high as 92%.

Short test period
It only takes 8 days to sort the drug efficacy and sensitivity of the drugs or combination regimens recommended by doctors, providing a scientific basis for the selection of individualized precision medicines for patients.

Low cost
New generation of drug susceptibility testing technology, which is faster and more economical, which costs 90% lower than traditional PDX.

Applicable to a wide range of samples
Surgical tissue, biopsy tissue, pleural and ascites samples, blood, and bone marrow are all suitable for MiniPDX™ drug susceptibility testing.

Originality
The first company to test drug susceptibility through tumor cell transplantation, MiniPDX™ drug susceptibility test is our patented product.

Preserves tumor biology
The tumor cells were directly transplanted into immunodeficient mice, thereby preserving the biological properties of the patient's original tumor.
Applications
At present, more than 2,400 cases of miniPDX testing services have been conducted, which not only provides precise medical guidance for patients, but also lays a data foundation for related clinical research.