平台介绍
上海立闻基于以MiniPDX为主的功能性检测平台,通过与临床专家的进行深层次的研究合作;目前已经发表学术论文多篇,其中以SCI为主.
上海立闻拥有着独特的转化医学平台,在临床研究和基础研究之间提供重要的媒介:一方面,我们基于MiniPDX的临床检测服务,可以协助临床医生进行以MiniPDX指导临床用药的临床研究的成果转化;另一方面,我们依托PDX和MiniPDX在内的功能性检测、多组学检测、及蛋白组学的综合性科研服务平台,可以助力基础研究的转化。
临床研究领域,涵盖多种瘤种的以MiniPDX为基础的精准治疗方案的对照研究、单臂研究等陆续发表,深化了MiniPDX的循证医学证据;此外,针对肿瘤精准治疗的其它领域/肿瘤类型,我们也期待进一步和各位专家进行合作。
基础研究领域,以PDX为代表的“金标准”药效研究手段在肿瘤研究领域受到了广泛的关注和应用,成为高水平成果转化的必要工具。MiniPDX由于其与PDX的一致性高,时间和价格上的优势,具备了部分替代PDX在基础科研的应用潜力,也得到越来越多专家的青睐。
学术成果汇总:
27.张菲菲,等. 用于预测癌症患者临床治疗方案的小鼠mini人源肿瘤异种移植模型的建立. 癌症. 2019; 4:165-176.
28.陈漪雯,等. OncoVee MiniPDX (TM)模型指导驱动基因阴性的晚期非小细胞肺癌患者化疗的疗效分析.实用癌症杂志. 2019; 8:1325-1327.
29.贾麟,等. MiniPDX模型在晚期肺癌伴恶性胸腔积液患者治疗中的指导作用. 中国医药导报. 2020;17(20): 131-134.
30.廖海燕,等. 间接体内Mini人源肿瘤异种移植模型再现转移性肠癌患者的药物治疗疗效. 肿瘤综合治疗电子杂志. 2020; 6 (3): 96-102.
31.周箴,等. 人源化免疫肺癌小鼠模型的建立及其在程序性死亡受体1抑制剂疗效评估中的作用. 上海交通大学学报(医学版). 2020,40(01): 37-43
应用实例
MiniPDX指导的术后用药方案可显著延长肝细胞癌患者的生存
近日,天津市第一中心医院张雅敏教授团队在权威杂志Cancer Chemotherapy and Pharmacology发表了题为《MiniPDX‑guided postoperative anticancer treatment can effectively prolong the survival of patients with hepatocellular carcinoma》最新的研究论文。该文章显示,利用MiniPDX模型药敏检测指导肝细胞癌患者肝部分切除术后的抗肿瘤用药可显著延长患者的生存期。患者中位总生存期从23.8个月延长至29.4个月;中位无病生存期从18.2个月延长至25.8个月。

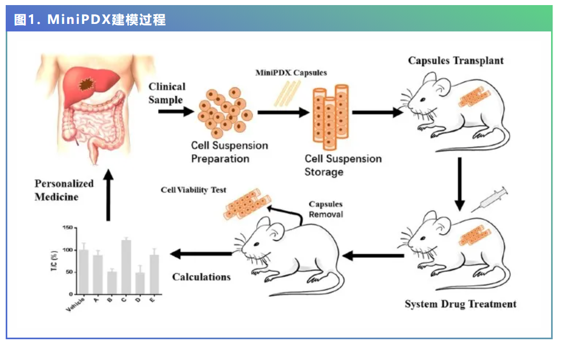
该研究入组了28例HCC患者,利用其肿瘤组织进行迷你人源性肿瘤移植动物模型(Mini patient derivedxenograft, MiniPDX模型)的建立,并分别测试了肝部分切除术后5组不同的预防用药方案 (5-FU+奥沙利铂、吉西他滨、乐伐替尼、瑞戈非尼、索拉非尼)作用下肿瘤细胞的增殖率,以筛选出患者最为敏感的药物方案。
研究者同时选择接受经验性用药方案索拉非尼进行治疗的42例HCC患者作为对照组;分析两组患者的无病生存期 (DFS)和总生存期 (OS);同时分析了药物敏感性与HCC有关的生物标志物 (包括AFP, Ki-67, VEGFR, FGFR, P53,和Nrf2)的相关性。
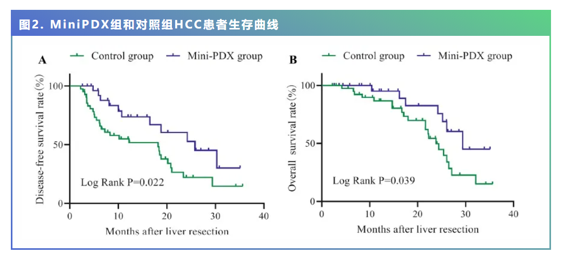
结果显示,最长随访36个月,使用MiniPDX筛选的用药方案进行治疗,HCC患者中位OS为29.4个月,明显长于使用传统用药方案患者的23.8个月 (P=0.039)。而使用MiniPDX用药方案的患者的中位DFS为25.8个月,同样明显长于使用传统用药方案的患者的18.2个月 (p=0.022)。
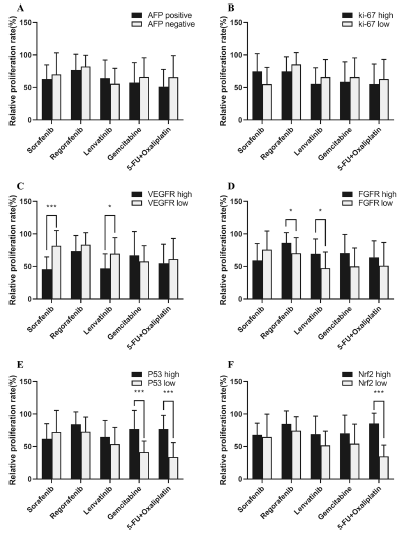
此外,研究人员还发现,不同药物敏感性与HCC生物标志物之间显示了一定的相关性:索拉菲尼和乐伐替尼在VEGFR高表达的患者中显示了更高的抗肿瘤活性;FGFR高表达的HCC患者对瑞戈非尼和乐伐替尼更加敏感;而P53高突变的患者一定程度表现出对细胞毒药物的耐受;Nfr2低表达的HCC患者中,5-FU联合奥沙利铂显示了更强的细胞毒性。


-227.jpg)
